Product Description
Ursodeoxycholic Acid
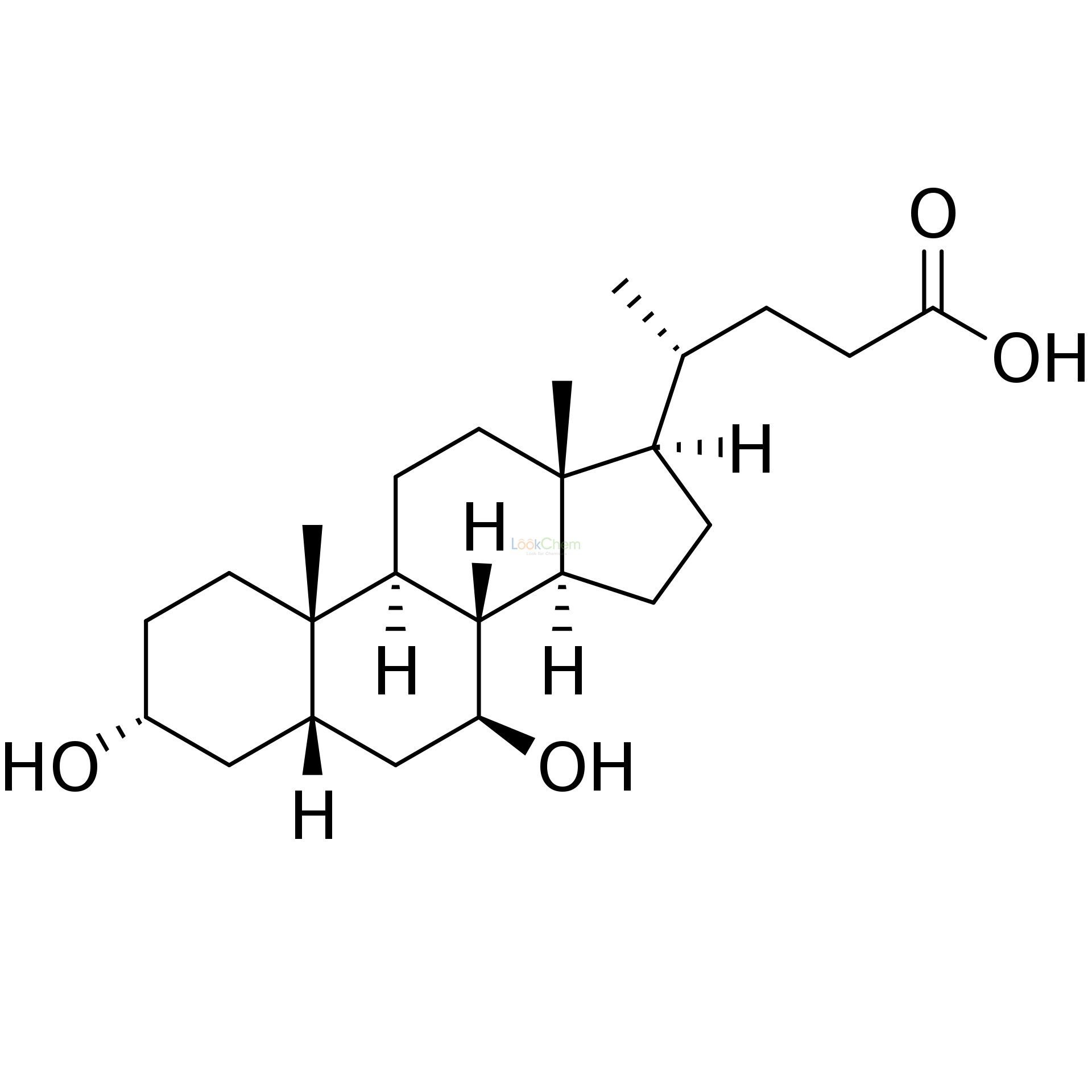
- 128-13-2
- C24H39O4
Brief Description
| Product name | Ursodeoxycholic acid |
| Synonyms | 3,7-Dihydroxycholan-24-oic acid; 3alpha,7beta-Dihydroxy-6beta-cholan-24-oic acid; Ursodiol; 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethanol; (3alpha,5beta,7alpha)-3,7-dihydroxycholan-24-oic acid; (3alpha,5beta,7beta)-3,7-dihydroxycholan-24-oic acid; (4R)-4-[(3R,5S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid; |
| MOQ | 25KG |
| CAS No. | 128-13-2 |
| Appearance | An almost white powder |
| Molecular Formula | C24H39O4 |
| Molecular Weight | 391.5646 |
| Assay | 99% |
| Application | Pharma grade or research purpose |
| Packing | As per your request |
| Storage | Preserve in tight, light-resistant containers in a cool place |
| Remarks | NA |
| Custom synthesis | Available |
| Supply Ability | 10000kg/month |
Medical use
An incomplete list of the current uses is as follows:
Reduction in gallstone formation, either in patients with gallstones unfit for cholecystectomy, or obese patients undergoing rapid weight loss to prevent gallstone formation.
For the treatment of primary biliary cholangitis (also known as primary biliary cirrhosis, PBC).
To aim to improve bile flow in patients with cystic fibrosis (controversial[5])
In newborn infants with impaired bile flow
After bariatric surgery, to prevent cholelithiasis due to the rapid weight loss with biliary cholesterol oversaturation and also biliary diskinesia secondary to abnormalities in cholecystokinin and biliary enervation.
Meta-analyses have borne out conflicting results on the mortality benefit of UDCA in PBC, however analyses that exclude trials of short duration have demonstrated a survival benefit and are generally considered more clinically relevant. A Cochrane systematic review in 2012 found no significant benefit in reducing mortality, the rate of liver transplantation, pruritus or fatigue. Ursodiol is the only FDA approved drug to treat PBC but many patients do not respond; other treatments are under study.
Ursodiol may be used to treat intrahepatic cholestasis of pregnancy, to relieve the symptoms of itching, to decrease infant mortality rate, and to decrease bile absorption. Ursodiol is not believed to reduce maternal mortality from hemorrhage in such cases.
In children, ursodeoxycholic acid use is not licensed, as its safety and effectiveness have not been established. Evidence is accumulating that ursodeoxycholic acid is ineffective and unsafe in neonatal hepatitis and neonatal cholestasis.
There is insufficient evidence to justify routine use of ursodeoxycholic acid in cystic fibrosis, especially that available data for analysis of long-term outcomes such as death or need for liver transplantation is lacking.
In double the recommended daily dose, ursodeoxycholic acid reduces elevated liver enzyme levels in those with primary sclerosing cholangitis, but its use was associated with an increased risk of serious adverse events (the development of cirrhosis, varices, death or liver transplantation) in patients who received ursodeoxycholic acid compared with those who received placebo. Serious adverse events were more common in the ursodeoxycholic acid group than the placebo group. The risk was 2.1 times greater for death, transplantation, or minimal listing criteria in patients on ursodeoxycholic acid than for those on placebo.
It is concluded that ursodeoxycholic acid use is associated with improved serum liver tests that do not always correlate with improved liver disease status. WHO Drug Information advises against its use in primary sclerosing cholangitis in unapproved doses beyond 13–15 mg/kg/day.
Recent research at the University of Sheffield indicates that Ursodeoxycholic Acid shows promise in the treatment of Alzheimer's Disease.
Our Factory:


